- AI Empowers the Entire Drug Development Pipeline! WMU Publishes Authoritative Review in Top-Tier Journal Nature Medicine
- Author:School of Ophthalmology and Ooptometry Date:February 7, 2025
Drug development is a complex and time-consuming endeavor that traditionally relies on the experience of drug developers and trial-and-error experimentation. The advent of artificial intelligence (AI) technologies, particularly emerging large language models and generative AI, is poised to redefine this paradigm. The integration of AI-driven methodologies into the drug development pipeline has significantly enhanced both the efficiency and effectiveness of this process.

Recently, a collaborative team comprising Prof. Zhang Kang's and Prof. Li Xiaokun’s groups from Wenzhou Medical University (WMU), and Prof. Yang Shengyong's group from Sichuan University, along with researchers from Macau University of Science and Technology, Sun Yat-sen University, Guangzhou National Laboratory, National Institute of Biological Sciences, and Stanford University, published a comprehensive review titled "Artificial Intelligence in Drug Development" in the prestigious international journal Nature Medicine. The article systematically reviews the advancements in AI applications throughout the entire drug development pipeline, encompassing disease target identification, drug discovery and design, synthesis planning, preclinical studies, clinical trials, and post-market surveillance. It highlights cutting-edge achievements of AI-augmented drug development and explores promising future research directions.
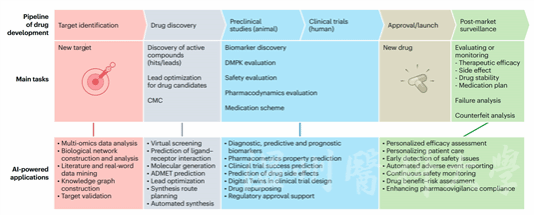
The review meticulously summarizes AI’s transformative roles across various stages of drug development, including biomarker identification, drug screening, drug design, and clinical trials. It emphasizes that AI not only improves R&D efficiency and reduces costs but also accelerates the translation of therapeutics from bench to bedside.
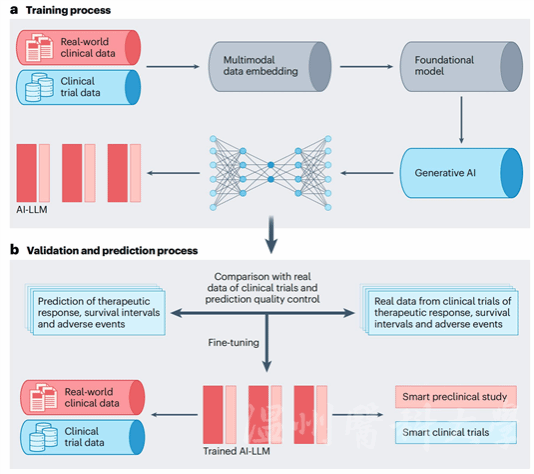
Prof. Zhang Kang (co-corresponding author) of WMU explained that AI optimizes clinical trials by analyzing patient data (including genetic information, medical history, and lifestyle). It identifies biomarkers and patient characteristics affecting drug response and further designs efficient trial protocols. By refining participant selection and outcome measurement, AI enhances clinical trial success rates and expedites drug development. The input of real-world data enables AI to predict adverse events and drug interactions.
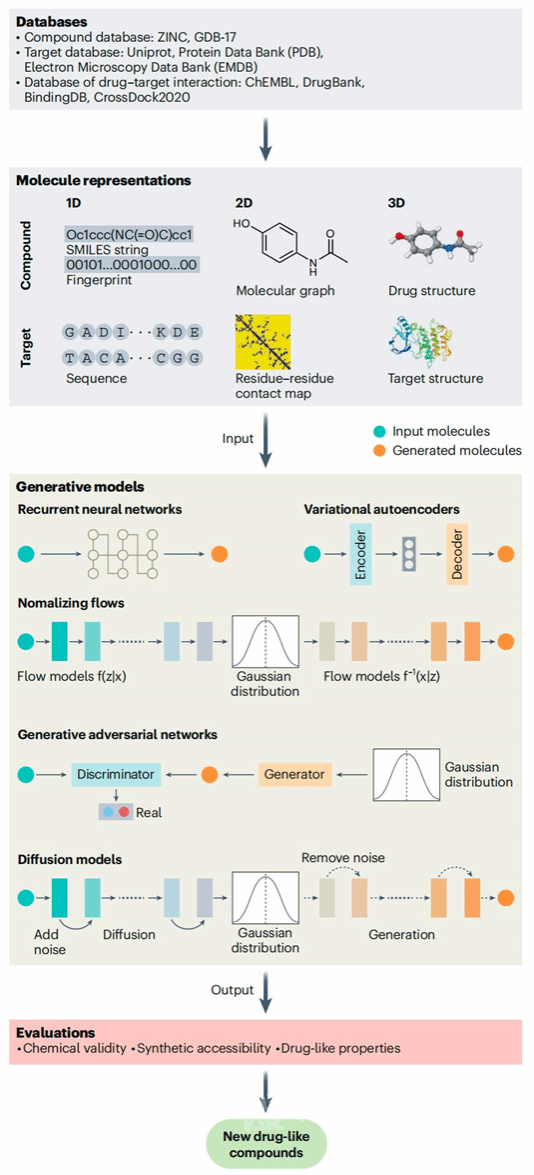
Professor Yang Shengyong (co-corresponding author) from Sichuan University noted that despite significant progress, AI-powered drug development faces challenges such as data scarcity, low model transparency, and high computational costs. As a result, no AI-developed drugs have entered Phase III trials yet. Future solutions include enhancing data sharing, developing sparse AI methods and multimodal pre-trained models, integrating multi-omics data, and incorporating physical laws to improve prediction accuracy and algorithmic transparency. In addition, collaboration with cloud service providers to develop efficient algorithms, refine clinical trial designs, and support precision medicine decisions will further accelerate the drug development process and benefit human health.
Nature Medicine invites globally leading scientists in their fields to provide review articles that drive the progress of the industry, predict the emerging trends, and establish best practice guidelines.
Article Link: https://www.nature.com/articles/s41591-024-03434-4
