- Cell Metabolism | Breakthrough: Huang Zhifeng’s research group at WMU identifies new therapeutic target for cholestasis
- Author:School of Pharmaceutical Sciences Date:October 24, 2024
Researchers from Academician Li Xiaokun’s team at Wenzhou Medical University, led by Professor Huang Zhifeng and Dr. Song Lintao, have achieved a significant breakthrough, with their study, titled “Hepatic FXR-FGF4 is required for bile acid homeostasis via an FGFR4-LRH-1 signal node under cholestatic stress”, published in the prestigious academic journal Cell Metabolism (IF=27.7).
The research identified hepatic Fgf4 as a direct farnesoid X receptor (FXR) target that paracrinally signals to downregulate Cyp7a1 and Cyp8b1. This liver-centric pathway acts as a first-line checkpoint for intrahepatic and transhepatic bile acid (BA) flux upstream of the peripheral FXR-FGF15/19 pathway, which together constitutes an integral hepatoenteric control mechanism that fine-tunes BA homeostasis, counteracting cholestasis and hepatobiliary damage. This work is a strong complement to the research on the FXR-FGF signaling axis in bile acid regulation over the past two decades, shedding light on potential therapeutic strategies for cholestasis diseases. This is another significant scientific discovery by Professor Huang Zhifeng’s research group, following the group’s discovery last year that a single central administration of FGF4 could elicit a sustained antidiabetic effect lasting more than seven weeks (Cell Metabolism, 2023, 35(6):1022-1037).

Bile acid is of great importance for the digestion and absorption of fat-soluble nutrients and acts as signaling molecules to adjust metabolism and energy homeostasis. Disruption of bile acid homeostasis or related signaling pathways can cause cholestasis, liver damage, metabolic disorders, and even liver cancer. Maintenance of bile acid homeostasis relies mainly on negative feedback regulation mechanisms and spatiotemporally controlled enterohepatic circulation. The regulation of bile acid synthesis mainly relies on the activation of FXR expressed in the liver and intestine, which acts as a bile acid sensor and controls the transcription of genes involved in bile acid synthesis, transformation, transportation, and signaling.
Previous studies have shown that activated FXR can improve transcription of SHP, MAFG in the liver, and FGF15/19 in the ileum under the condition of increased postprandial intestinal bile acids. Intestinally-secreted FGF15/19 selectively binds to and activates the hepatic FGF receptor 4 (FGFR4)-β-klotho (KLB) complex, and represses the transcription of Cyp7a1 and Cyp8b1, genes encoding for enzymes critical for bile acid synthesis, in a signal pathway that has not yet been completely understood. This endocrine pathway combined with the physiological response to food digestion, preventing new bile acid synthesis postprandially, is a necessary physical need to prevent the potential toxicity of excess bile acids to the hepatobiliary and intestinal systems. Because the ileum is separated from the liver, which is the main organ for bile acid synthesis, the function of the ileum FGF15/19 pathway lags the role of liver in early postprandial bile acid synthesis. Such restriction of space and time raised an essential question of how intrahepatic bile acid synthesis and the total bile acid content is controlled in the liver before the endocrine FGF15/19 signaling pathway works.
To answer this question, the research group utilized FGF15 knockout mice. The results showed that FXR, when activated, could still maintain repressive control over Cyp8b1 expression in the absence of FGF15, but not Cyp7al. Interestingly, such repression effect was completely lost in the livers of FGFR4-deficient mice. The researchers, therefore, proposed an important hypothesis that there is another FXR-controlled member of the FGF family in the liver, that directly activates hepatic FGFR4 to inhibit bile acid synthesis via autocrine or paracrine pathways, particularly through repressing Cyp8b1 pathway.
To validate the hypothesis, the research group discovered that hepatic FGF4 is another direct target gene of FXR through systematic screening analyzing FGF family members responsible for such differences in FXR’s effects. As a signal mediator of hepatic FXR, FGF4 is able to suppress the transcription of Cyp7a1 and Cyp8b1, particularly Cyp8b1, thereby inhibiting hepatic bile acid synthesis and improving cholestasis. Further mechanistic study found that this liver-centric action of FXR-FGF4 is mediated by an FGFR4-LRH-1 signaling pathway which had not been reported: upon activation of FGFR4 by FGF4, the nuclear receptor LRH-1 in cytoplasm is recruited to the kinase domain, leading to phosphorylation-mediated inactivation and loss of LRH-1’s targeting impact to Cyp7a1 and Cyp8b1 gene promoters. Crucially, the findings were validated in clinical patients, exhibiting reduced hepatic expression of FXR and FGF4 and impaired FXR-FGF4 signal axis compared to healthy controls.
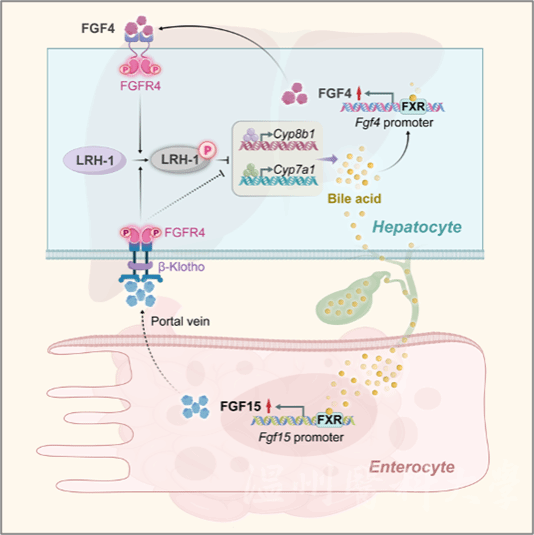
Figure 1. Schematic illustration of the FXR-FGF4-FGFR4-LRH-1 signaling pathway regulating bile acid synthesis
In conclusion, the research has identified hepatic FGF4 as a direct transcriptional target of FXR and a liver-centric feedback suppressor of bile acid synthesis upstream of the peripheral FGF15/19 pathway. Spatiotemporally controlled bile acid synthesis is of significance to the maintenance of bile acid homeostasis in response to a variety of physiological needs and pathological challenges. The elucidation of the hepatic FXR-FGF4 to FGFR4-LRH-1 pathway clarifies and expands the mechanism and mode of precise spatiotemporal control. Relevant findings have provided novel mechanistic insights into hepatic bile acid synthesis and enterohepatic/systemic bile acid homeostasis regulation. The discovery also has opened a new door to the design of potential therapeutic strategies for the treatment of cholestasis and associated diseases.
Professor Huang Zhifeng, Academician Li Xiaokun, and Dr. Song Lintao contributed equally to this work as corresponding authors; Dr. Song Lintao, Postdoctoral Fellow Hou Yushu, and postgraduate student Xu Da are the first authors. This study was funded by projects including the Major Research Program Integration Project of the National Natural Science Foundation of China, the Major Project of the Natural Science Foundation of Zhejiang Province, and the Top Talent Program of Oujiang Laboratory.
The research received commentary from two well-known experts in related fields.
