- Academician Song Weihong’s Team at WMU Reveals New Pathogenic Mechanism of CNTNAP2 in ASD
- Author:Center of Excellence Date:June 23, 2023
On June 5, Professor Song Weihong, fellow of the Canadian Academy of Health Sciences and academic vice president of WMU, and his team published a research paper titled “CNTNAP2 intracellular domain (CICD) generated by γ-secretase cleavage improves autism-related behaviors” in leading international journal Signal Transduction and Targeted Therapy (IF = 38.104) together with Professor Li Jiada’s team from the Center for Medical Genetics of School of Life Sciences at Central South University. The paper reveals the molecular pathological mechanism of autism spectrum disorders (ASD) caused by the deletion/mutation of contactin-associated protein-like 2 (CNTNAP2), an ASD risk gene, providing novel therapeutic implications for the drug development of ASD.

As the most prevalent neurodevelopmental disorders in children, ASD are characterized by clinical syndromes such as deficits in social interaction, language development, and inflexible interests or repetitive behaviors. The pathogenesis of ASD remains unclear. CNTNAP2 is a widely validated ASD risk gene. Cntnap2-deficient (Cntnap2-/-) mice also show core autism-relevant behaviors. Belonging to the Neurexin superfamily, CNTNAP2 is a typical multidomain type I transmembrane protein localized in the post-synaptic region of neurons. By sequence analysis, the study found that the amino acid sequences of the transmembrane domains of CNTNAP2 and amyloid-β precursor protein (APP) were highly similar, and a motif within the transmembrane domain of CNTNAP2 was highly homologous to the γ-secretase cleavage site of APP (VVIF / VVIA, Fig.1). In addition, CNTNAP2 could interact with proteolytic enzymes of the ADAM family (ADAM22, ADAM23, ADAM11), members of the LGI family, and MAGUK proteins (DLGs and MPPs), suggesting that CNTNAP2 may undergo proteolytic cleavage to release extracellular and intracellular domains to perform biological functions.
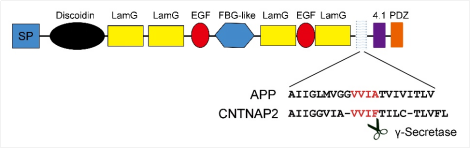
Fig.1 The basic structure of CNTNAP2 and the transmembrane amino acid sequence of APP
Through systematic analysis, it was found that CNTNAP2 underwent two steps of protease-mediated cleavage. The first cleavage in the extracellular juxtamembrane region released a soluble extracellular fragment and a membrane-tethered C-terminal fragment (CTF). The CTF was further processed by γ-secretase to generate the CNTNAP2 intracellular C-terminal domain (CICD). Besides, overexpression of CICD induced by stereotaxic injection of adeno-associated viruses into the medial prefrontal cortex (mPFC) of mice normalized the deficit in the core autism-relevant behaviors of Cntnap2−/− mice, including social deficit and repetitive behaviors. Further study identified that CICD may regulate the expression of the downstream genes such as Necdin through regulating the nuclear entry of some transcription genes (such as CASK ), thereby affecting the development and electrical activity of neurons and participating in the regulation of the autism-relevant behaviors. The study is the first to reveal the critical role of CICD generated by proteolytic cleavage of CNTNAP2 in autism-relevant behaviors.
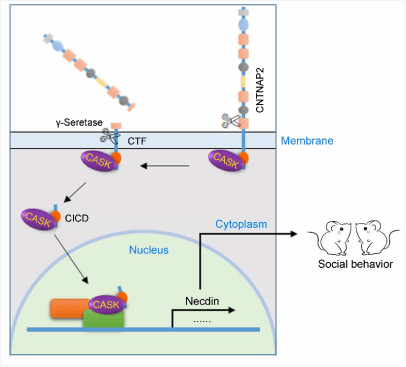
Schematic presentation of a proposed mechanism of CNTNAP2 in autism-relevant behaviors
Academician Song Weihong at WMU and Professor Li Jiada from the Center for Medical Genetics at Central South University are the co-corresponding authors of the paper. Teacher Cai Fang from Academician Song’s team and PhD Student Zhang Jing from Professor Li’s group who performed most of the experiments are the co-first authors.
Text translated by Wang Xinyue and reviewed by Sun You
